Spravato (esketamine) has emerged as a groundbreaking treatment option for individuals suffering from treatment-resistant depression (TRD). Approved by the FDA, this nasal spray represents a significant shift in how depression is managed.
In this article, we’ll explore what Spravato is, how it works, its cost, side effects, and other key details to help you understand this innovative therapy.
What is Spravato?

Spravato is the brand name for esketamine, a chemical derived from ketamine. Administered as a nasal spray, it is specifically approved for adults with treatment-resistant depression. Unlike traditional antidepressants, which can take weeks to show effects, Spravato works rapidly, offering relief to patients who have not responded to other treatments.
How Does Spravato Work?
The mechanism of action for Spravato involves targeting the N-methyl-D-aspartate (NMDA) receptor in the brain. By modulating glutamate, a neurotransmitter involved in mood regulation, Spravato promotes synaptic growth and enhances neural connectivity. This mechanism is distinct from conventional antidepressants, which primarily influence serotonin, dopamine, or norepinephrine pathways.
Research has shown that Spravato can reduce symptoms of depression within hours, making it a game-changer for patients in crisis or those who have tried multiple medications without success.
FDA Approval and Regulation
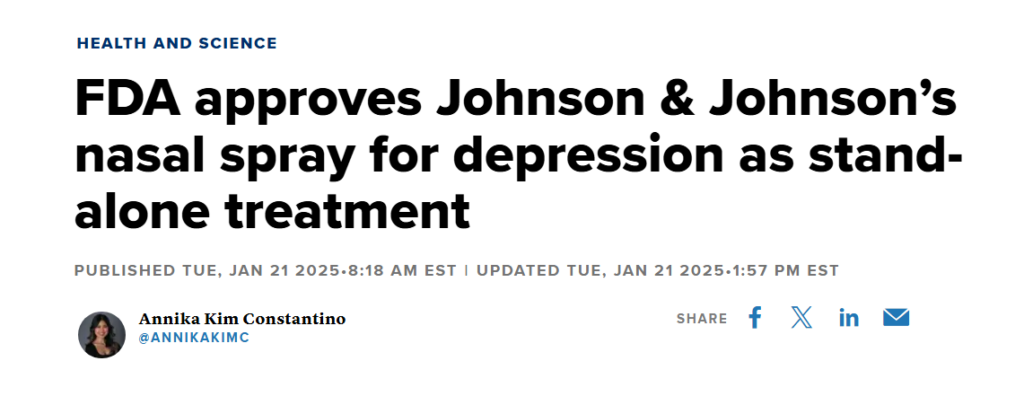
Spravato received FDA approval in March 2019 for use in conjunction with an oral antidepressant. Due to its potential for misuse, it is classified as a controlled substance and is only available through the Spravato REMS (Risk Evaluation and Mitigation Strategy) program, which ensures its safe administration in certified healthcare settings.
In addition, the FDA approved Spravato for patients experiencing acute suicidal ideation, marking the first nasal spray approved for this specific purpose in mental health treatment.
Who Can Use Spravato?
Spravato is indicated for:
- Adults with treatment-resistant depression.
- Patients with major depressive disorder (MDD) and acute suicidal ideation (approved under strict medical supervision).
Exclusion Criteria: Spravato is not recommended for individuals with:
- Uncontrolled high blood pressure.
- A history of aneurysms or brain bleeding.
- Severe liver impairment.
- Known hypersensitivity to esketamine or ketamine.
How is Spravato Administered?
Spravato is a nasal spray that must be administered under the supervision of a healthcare professional. The process typically involves the following steps:
- The patient self-administers the spray under guidance.
- Observation for two hours in the treatment center to monitor for side effects.
- Regular sessions are scheduled, starting twice weekly and tapering off over time.
The treatment schedule typically spans several weeks, with gradual reductions in frequency as patients respond to the therapy.
Spravato Treatment Cost
The cost of Spravato can vary depending on the treatment center, insurance coverage, and dosage. On average, the price for each session ranges from $600 to $900, with total costs reaching $4,720 to $6,785 for the first month. Many insurance providers cover Spravato, but patients should verify coverage and co-payments in advance.
Financial assistance programs may be available through the manufacturer, Janssen Pharmaceuticals, to help eligible patients reduce out-of-pocket costs.
Spravato Side Effects
Like any medication, Spravato can have side effects, which may include:
- Dizziness
- Dissociation (feeling detached from reality)
- Sedation
- Increased blood pressure
- Nausea and vomiting
- Headache
Rare but Serious Side Effects:
- Severe blood pressure spikes.
- Allergic reactions.
Patients are monitored closely after administration to manage any adverse effects effectively. Healthcare providers also educate patients on what to expect during treatment sessions.
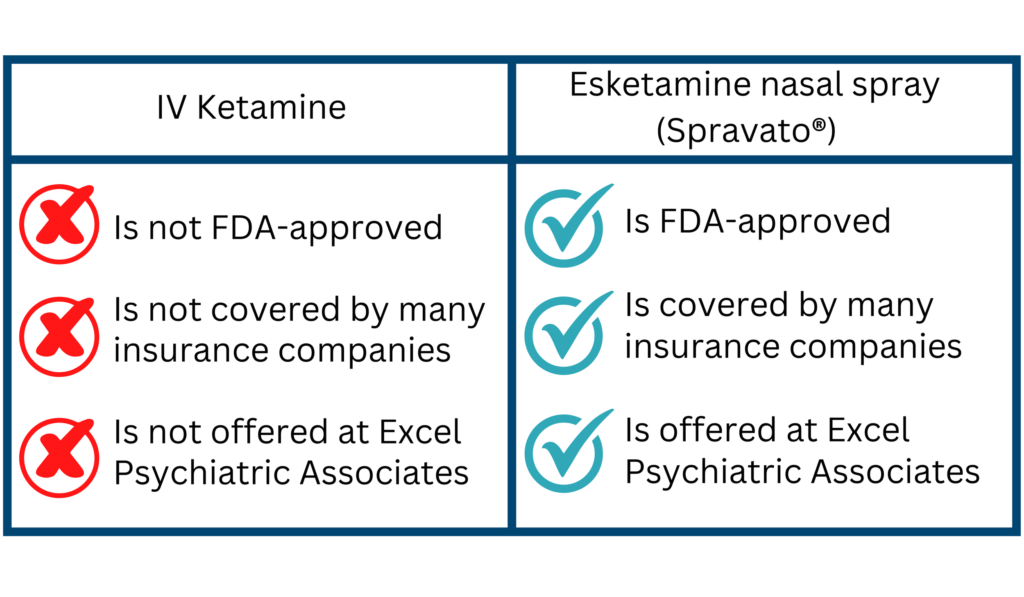
Comparing Esketamine and Ketamine
While both esketamine and ketamine are derived from the same compound, esketamine is a more refined version, designed for nasal administration and FDA-approved for depression. Traditional ketamine, often administered intravenously, is used off-label for depression but lacks FDA approval for this indication.
Key Differences:
- Administration: Spravato is a nasal spray, while ketamine is usually given intravenously or intramuscularly.
- Approval: Spravato is FDA-approved for TRD; ketamine is not.
- Cost: Ketamine treatment is often less expensive but may not be covered by insurance.
How to Access Spravato Treatment
To receive Spravato, patients must:
- Obtain a prescription from a qualified healthcare provider.
- Locate a certified Spravato Treatment Center near them.
- Enroll in the Spravato REMS program to ensure safe and monitored administration.
To find a treatment center, patients can use online resources provided by Janssen Pharmaceuticals or consult their healthcare provider for recommendations.
Spravato Reviews and Patient Experiences
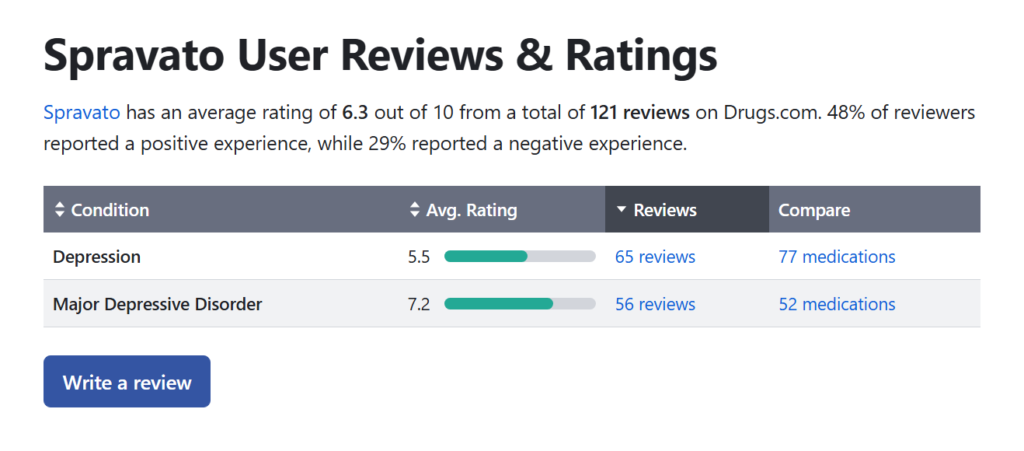
Patient reviews of Spravato are mixed but generally positive for those with treatment-resistant depression. Many report significant improvements in mood and functionality within hours of administration. However, some patients experience side effects that can interfere with daily activities, emphasizing the need for careful monitoring.
70% of patients reported a noticeable reduction in depressive symptoms within the first four weeks of treatment.
The American Journal of Psychiatry
Conclusion
Spravato offers a promising option for individuals who have not found relief with traditional antidepressants. While its cost and administration requirements can be barriers, the potential benefits for treatment-resistant depression are substantial. Patients interested in Spravato should consult their healthcare provider to discuss eligibility and treatment options.
Disclaimer
This article is for informational purposes only and does not constitute medical advice. Always consult a qualified healthcare provider to determine whether Spravato is the right treatment option for you. you can follow us on our website digitecultra.io
FAQs